Medical Device
Quality
Management
Consultancy,
Services And Systems
Working with medical device companies to enhance and implement quality management systems and processes
Comply
Effectively
with
A quality eco-system enabling compliance, efficiency and higher quality medical devices
A key part of regulatory compliance in EU, USA and worldwide is the establishment of a Quality Management System. MedQtech can help you develop your existing quality management system to comply to medical device regulations and standards (such as QSR and ISO13485), can focus on specific areas in your quality system, or can help you build and implement a full new quality management system.
Utilize our experience to quickly develop efficient and compliant quality management processes and build compliant technical documentation for your medical device. Feel confident that all necessary steps to get your CE-mark and successfully register your medical device are completed.
Quality management system implementation
ISO13485 certification and QSR readiness
Facilitation of ISO14971 risk management
Act as your quality and regulatory resource
Develop/maintain QMS processes
Project management
Get started with your QMS simply and effectively
We can adapt the implementation of a quality management system to the needs of your organization. Work with us to ensure the focus is correct, as a small start up innovation company or a large global organization setting up a new product line or department, your needs are very different.
We make sure the core processes are put in place in the order that meet our customers’ requirements. We could begin with your design and development processes, supplier control or focus on manufacturing – we have the experience to ensure you get the maximum results for your business needs.
Utilising your QMS to drive company wide improvement
With many medical device companies the primary reason for their QMS commitment is to get regulatory compliant. Our experience has shown that by implementing a QMS solution effectively your business will benefit across the organisation.
With our support in training and knowledge extension the QMS system can spread good practice and high quality effectively, saving costs and increasing quality results. Maximise your QMS investment by working with MedQtech.

“We have worked closely with MedQtech to enable our team with a new quality management system. There is no doubt that MedQtech have accelerated this process, and ensured the team have hit a high level of enablement helping across the business”
Medical device company
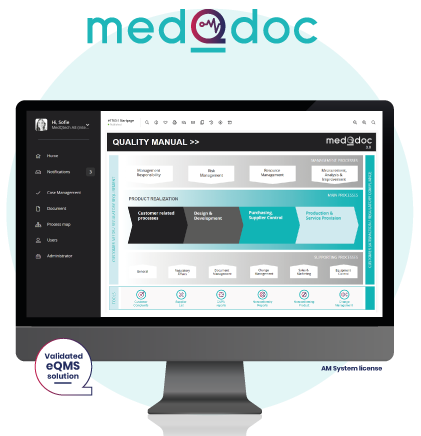
MedQdoc Quality Management System (eQMS)
We work with several industry eQMS software solutions, but many of our customers use our own eQMS solution MedQdoc.
- Over 130 ready-to-use templates
- Simple step-by-step workflow
- Designed by compliance experts
- Cloud based flexibility
- Accelerate your medical device compliance while enhancing your quality management system.
Accelerate your medical device compliance while enhancing your quality management system.