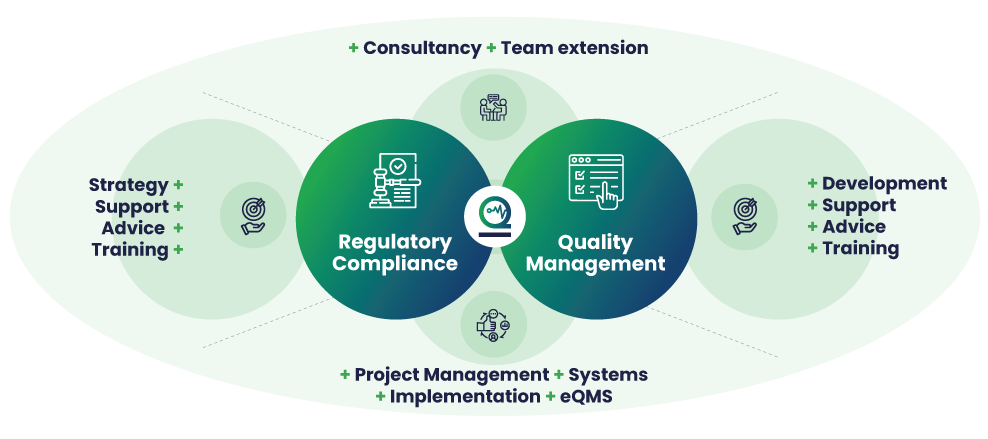
5 advantages of an effective eQMS for Medical Device Companies
Having read the MedQdoc blog post on “Five Ways MedQDoc Adds Value for Medical Device Distributors”, we wanted to comment on the topic from a compliance consultant’s point of view. Read our thoughts on the MedQdoc blog post from and how an effective eQMS can improve your business operations.