Labelling requirements in MDR
MDR lists some requirements for medical device labelling and instructions for use (IFU). Although they are requirements, you can also see them as guidance, helping you to not forget anything necessary or interesting for the user. This blog article gives a short introduction emphasizing some of the essentials in the regulation.
Where to look
In the Medical device regulation (MDR), Annex I lists the requirements for the label and IFU. The Annex lists 19 items essential for the label and 28 for the IFU. Don’t be frightened if it sounds like a huge amount. Many of them are not applicable for your product.
Product standards: For many medical devices there are international or European product standards that list requirements for the performance of the device. Even though this is not the main objective for these standards, they can also have requirements for the label and/or instructions for use.
Other standards: ISO 20417:2021 describes the information from the manufacturer to be supplied to the user. There is also a symbol standard, ISO 15223-1:2021, listing all harmonized symbols that can be used by the manufacturer.
All of the above will be explained in detail below.
MDR medical device label
As described above, there are 19 items to consider regarding the label. 19 sounds a lot but many of them are rather obvious and some are not applicable for all devices. The most relevant are:
- Name and address of the manufacturer. Sounds obvious but there are some things to consider. The address needs to be a physical place; a PO box is not enough. The reason is of course that you must be able to find the actual place where the company is situated.
- Special storage or handling conditions. It’s not enough to write these in the IFU, it must be stated on the label. Note the wording “special”, ambient temperature does not need to be addressed.
- If the device is for single use.
- If the device is supplied sterile, it must be noted on the label, together with the sterilisation method.
 Indication that it is a medical device. This is a new requirement compared to the previous directive (MDD). The simplest way to solve this is to use the standardized symbol for a medical device: A square with MD in capital letters.
Indication that it is a medical device. This is a new requirement compared to the previous directive (MDD). The simplest way to solve this is to use the standardized symbol for a medical device: A square with MD in capital letters.
MDR Instructions for use (IFU)
28 requirements are listed for the IFU. When it comes to medical device labelling not all requirements are applicable for all devices. Below you can find the most general requirements that must be stated in the IFU:
- Risks, contra-indications and undesirable side-effects.
- Date of issue of the IFU and revision number if it has been revised.
- A statement that serious incidents shall be reported, i.e. that they shall be reported both to the manufacturer and the competent authority of the country where the user is established.
- Warnings and precautions for safe disposal.

Product standards
Fulfilling product standards is one way of showing compliance to MDR. Depending on the product, there are different numbers of standards to follow, and the standards often refer to other standards making it grow even more. Complex products can have plenty of standards, not just in the medical device field, but also in other areas, e.g. emitting radiation.
The more standards to follow the more requirements on medical device labelling. There are manufacturers that need to consider more than 100 requirements. Make sure you have control of the labelling requirements of your type of product.
Other standards
a) ISO 20417:2021, Medical devices – Information to be supplied by the manufacturer.
The standard makes a generic overview for all medical devices. Amongst other things it gives recommendations on how to test the legibility and durability of the label. The requirements for the label and IFU are derived from MDR, Annex I.
b) ISO 15223-1:2021 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied.
The standard lists all recognized symbols to be used on the label and in the IFU. There are symbols representing “Manufacturer”, “Expiry date”, “Sterilization method” and many others. By using the symbols from the standard, you don’t need to worry about translations on the label and can use the same label for all markets. Examples of symbols:

Manufacturer
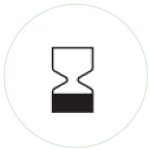
Use by date

Sterilized using ethylene oxide
Other things to consider
Translations! Most European countries require the label and IFU to be written in their language. By using harmonized symbols from standard ISO 15223-1:2021 you rely on the symbols instead of text and translation is not necessary.
Where to put the label? The main rule is that the label shall be put on the device. If the device is too small to bear a label, it is enough to put the label on the packaging. The label shall be easily legible, so don’t hide it behind a lid or similar.
Do you need more help with complying to
medical device labelling requirements?
Don’t hesitate to contact us at MedQtech, we have extensive experiences from working with labelling for all types of medical devices and we would be happy to help you out!




