Are you planning to, or in the process of, developing a medical device but aren’t sure how to classify your device according to MDR 2017/745? Or maybe you haven’t had the time to really get to understand the classes and classification rules yet.
Don’t worry, in this blog post you will get an overview of the MDR medical device classes and classification rules, and hopefully some inspiration on where and how to start.
So, why is the class of our medical device important? Because that is what tells us what actions we need to take before placing our product on the market and start selling it. Most of the results of these actions are going to end up as parts of the technical documentation sent to our Notified Body (MDR 2017/745, Annex II); our proof of the safety of our device. And the higher the class of our medical device, the higher the risk, so the more proof we need to provide.
Overview of the different medical device classes
Classifying your medical device can seem like an overwhelming task, especially if you have a more advanced product. However, if you read through MDR 2017/745 and all relevant MDCG documents, you will find a lot of information helping you to set the correct classification.
Easy right? OK, we understand, there is a lot of text to go through. That is why we have created this overview for you.
To start with, medical devices are divided into four classes according to EU MDR 2017/745:
- Class I
- Class IIa
- Class IIb
- Class III
Class I is for low-risk products and Class III for the highest risk products (MDR 2017/745, chapter V, section 1, article 51).
Examples of medical devices considered as Class I are bandages and wheelchairs – the consequences for the patient/user if something goes wrong are normally not severe and the risk of something going wrong when using the product is low.
An example of a medical device considered as Class III is a pacemaker – the consequences for the patient/user if something goes wrong could be very severe, even life-threatening and the potential for something to go wrong when using a complex device the product is much higher, relatively speaking.
Regarding Class I, they can be divided into two subclasses:
- Class I: A non-sterile device that does not have a measuring function and is not reprocessed
- Class Is/Im/Ir: A device that is delivered sterile (Is), or has a measuring function (Im) or is reprocessed (Ir)
Normally, a notified body (NB), does not need to certify a Class I product. However, for the second sub-classes above (Class Is, Im and Ir), an NB has to be involved.
But how do you figure out the correct classification for your product?
What do you need to know beforehand?
As in all aspects of life, you need to plan before you start executing. First of all, you need to know the intended purpose of your product before you begin. The intended purpose is explained by MDR as “The use for which a device is intended according to the data supplied by the manufacturer.” (MDR 2017/745, chapter 1, article 2, section 12). In other words, how and for what do you intend it to be used?
Furthermore, you will need to know the duration of use for your product (MDR 2017/745, annex VIII, chapter 1, section 1):

‘Transient’
means
“normally intended for continuous
use for less than 60 minutes.”
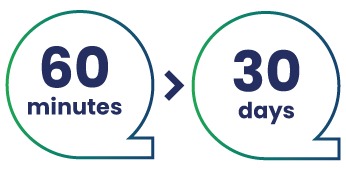
‘Short term’
means
“normally intended for continuous use
for between 60 minutes and 30 days.”

‘Long term’
means
“normally intended for continuous
use for more than 30 days.”
Also, you need to know if your product is invasive and/or active (MDR 2017/745, Article 2, point 4 and 6):

An active device means
“any device, the operation of which depends on a source of
energy other than that generated by the human body for
that purpose, or by gravity, and which acts by changing the density of or converting that energy.”
An invasive device means
“any device which, in whole or in part, penetrates inside the body, either through a body orifice or through the surface of the body.”
And lastly, there are also implementation rules which explain the classification under certain circumstances, e.g. if your product is used in combination with another medical device, or if your product contains software (MDR 2017/745, annex VIII, chapter 2).
Classification rules
So, finally we come to the part where we actually find out which class our medical device will have.
The classification rules are divided into:
- Non-invasive devices
- Invasive devices
- Active devices
- Special rules
Each classification rule under the above rulesets defines the applicable types of products and to which class it should belong (i.e. I, IIa, IIb or III) for the specific rule.
Example: Band-aid
For example, a band-aid is a non-invasive device, so we should look for the applicable rule under Non-invasive devices.
There we find rule 4, which seems to fit.
But then it gets trickier; we need to be sure about our intended purpose.
It could end up in different classes depending on whether our band-aid is just used to cover abrasions (and so a Class I device) or used on deeper cuts that need stitching (and so a Class IIb device).
(MDR 2017/745, annex VIII, chapter III, section 4.4.)

Example: Blood pressure monitor
Example: Blood pressure monitor
Another example is a blood pressure monitor that
contains sensors, a tablet for presenting results and
software that process the input data and calculates
the results. This device is therefore made up of three
different parts: sensors (disposables), hardware
(tablet) and software.
Clearly, this is an active device, so we have to look for the appropriate rules. Rule 10 applies to “Active devices for diagnosis and monitoring or intended for diagnostic or therapeutic radiology” (MDR 2017/745, annex VIII, chapter III, section 4.4.)
If we also use the document “MDCG 2021-24 Guidance on classification of medical devices” and look a little bit closer at Rule 10, we can actually find “Electronic blood pressure measuring equipment” as an example of devices “intended to allow direct diagnosis or monitoring of vital physiological processes”.
And voilá, this tell us our medical device should be Class IIa.
Is it really this easy? Well, not always. Following the steps above will definitely help you find the right class for your product, but sometimes it can be a little more complex, for example if you have more advanced devices, that are based on new technology or borderline products (i.e. is it a medical device or something else?). There might also be additional legislation, directives and standards to consider for certain medical devices.
One area we will cover in a future blog post is medical device software, which is becoming more relevant in the future, with the continual development of, for example, medical device AI-based tools and apps. This is an entire subject in itself and deserves its own blog post, so keep an eye out for it.
As explained in the beginning of this blog post, the class of your medical device determines what Technical Documentation to provide to your NB for product certification. So, how should you go about this? Don’t worry, we will cover that in a blog post soon.
It is always a good idea to reach out to experts in order to classify your medical device. This will usually save you both money and time. Our medical device compliance experts at MedQtech are ready, should you need any help. So, don’t hesitate to contact us!




